New Publication
My First Paper
I’m pleased to announce that my first paper with myself as the first author has been officially published as of May 15th. Click on the link to see the official journal page with the paper, Sulfate Recognition in Water via Charge-Assisted Hydrogen Bonding. If you can’t access the full paper, go to the bottom of this page for a download. The work for this paper was mostly completed early last year using a simple anion-binding receptor. In this post, I will provide a summary of the key points of the paper for those unfamiliar with the field of supramolecular chemistry.
In this paper, we have shown that charge and strong hydrogen bonding functional groups work together to greatly improve anion binding in water to overcome anion solvation barriers and demonstrates each’s contributions. This is impactful because water is the biologically relevant solvent and is highly comptetitive with receptors because water molecules easily form hydrogen bonded solvation layers around both the anion (the “guest”) and hydrogen binding groups on the molecule we made (the “host”). These solvation energy barriers must be overcome for binding to be observed, and is harder to overcome when the guest is very charge dense, because this property makes it highly solvated. Because we have the charge and the strong hydrogen bonding motifs, we are able to get selectivity for strongly solvated anions, like sulfate (SO42-) vs weakly solvated, more hydrophobic anions using this simple receptor. This selectivity is the opposite of what is normally achieved in this field, because a hydrophobic molecule, by definition, interacts weakly with water. The reason for this observed selectivity is most likely due to the charge complementarity as our host has 2+ charge and binds best with 2- charged guests.
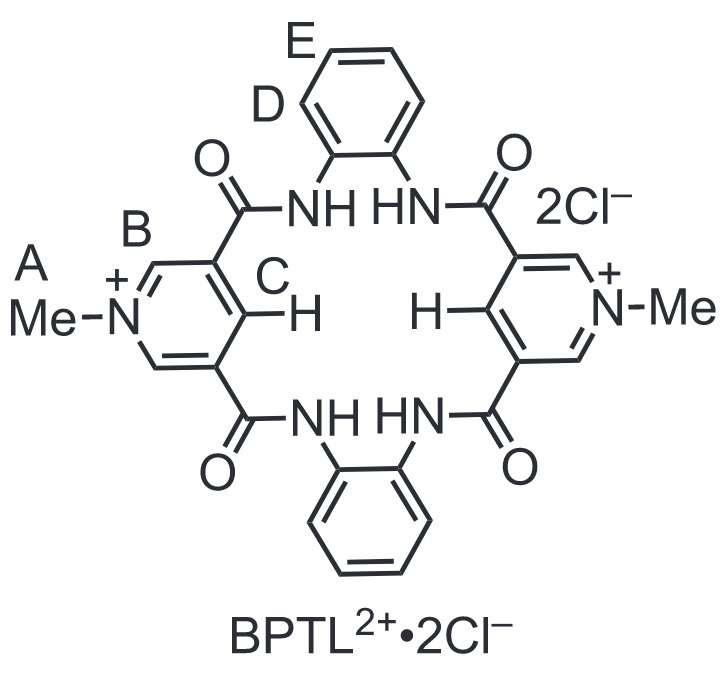 Chemdraw Structure of BPTL2+•2Cl-
Chemdraw Structure of BPTL2+•2Cl-
This receptor is a tetralactam macrocycle which features 4 amide bonds, 2 charged pyridinium units, and polarized carbon-hydrogen bonds which are involved in the anion binding. Based on these features, the molecule is dubbed our bipyridinium tetralactam (BPTL2+•2Cl-) macrocycle. The amide bonds point towards the center of the molecule the the pyridinium units polarize the C‒H opposite the nitrogen which also provide hydrogen bond donor convergent on the center. These convergent groups form a well-defined hydrogen bonding cavity for the anions to slot into. Moreover, the positive charge of the two pyridiniums electrostatically attract the anions. For these structural reasons, we rationalize why we observed strong binding with strongly solvated, charge dense anions. Namely, with sulfate (SO42-) and oxalate (C2O42-).
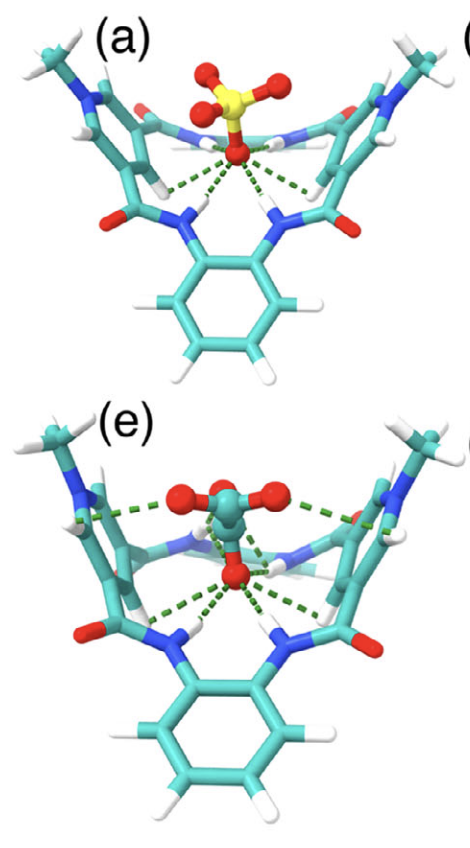 Computed Structure with SO42- (top) and C2O42- (bottom)
Computed Structure with SO42- (top) and C2O42- (bottom)
Based on crystal structures of the precursor molecule with bound guests as well as DFT optimized conformational isomers of the final products we see that the macrocycle forms a ‘saddle’ structure. In the precursor, it is clear that the groups central cavity are involved in forming 6 hydrogen bonds with the guests. In a simulated watery environment, the computed structures show the positively charged pyridinium units envelop the guests to interact with the negative charges and create a hydrophobic pocket for the guest which ‘squeeze’ out water molecules to desolvate them. These binding modes agree with the experimental observations which we’ll get into next.
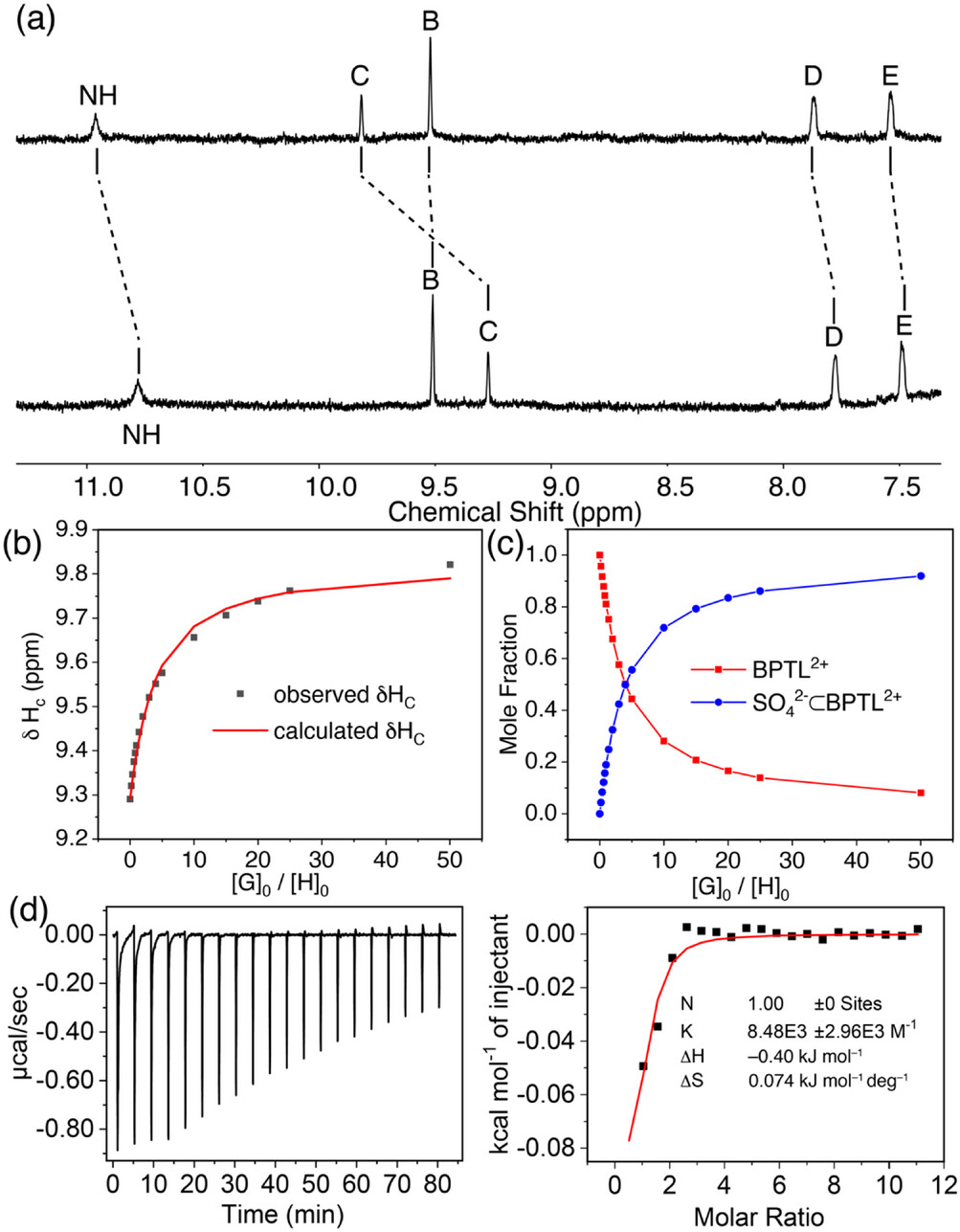 (a) ¹H NMR spectra (600 MHz, 10% D₂O / 90% H₂O) of solutions containing BPTL2+·2Cl⁻ (0.1 mM) with only (bottom) HCl (1 mM) and (top) with HCl (1 mM) plus Na₂SO₄ (5 mM). Proton labels are displayed in Figure 1. (b) Nonlinear fitting of the chemical shift changes of proton C during the titration of BPTL2+·2Cl⁻ with Na₂SO₄ in D₂O. (c) Calculated mole fraction changes of free BPTL2+ and the sulfate complex (SO₄2-⊂BPTL2+) in D₂O as a function of the guest–host mole ratio. (d) ITC profile of BPTL2+·2Cl⁻ (0.8 mM) during titration with Na₂SO₄.
(a) ¹H NMR spectra (600 MHz, 10% D₂O / 90% H₂O) of solutions containing BPTL2+·2Cl⁻ (0.1 mM) with only (bottom) HCl (1 mM) and (top) with HCl (1 mM) plus Na₂SO₄ (5 mM). Proton labels are displayed in Figure 1. (b) Nonlinear fitting of the chemical shift changes of proton C during the titration of BPTL2+·2Cl⁻ with Na₂SO₄ in D₂O. (c) Calculated mole fraction changes of free BPTL2+ and the sulfate complex (SO₄2-⊂BPTL2+) in D₂O as a function of the guest–host mole ratio. (d) ITC profile of BPTL2+·2Cl⁻ (0.8 mM) during titration with Na₂SO₄.
We did NMR titration studies and Isothermal Titration Calorimetry (ITC) to determine the binding affinity toward specific anions. Using proton (1H) NMR, the electron density around each proton can be elucidated. The protons which are involved in binding a negative charge will become more positively charged and move “downfield” to higher chemical shifts (ppm). The binding events are a fast equilibrium process where we observe the average chemical shifts of the hosts with and without the guest bound. As there is a larger excess of the guest, there will be a larger ratio of the host with the bound guest until eventually the host is saturated and adding more guest has no effect on changing the chemical shift. In our studies in deuterated water (D2O), we observed the signals which shifted the most are the N-H (the amide) protons, protons C (the other central protons) and protons B (adjacent to the charged nitrogen). This tells us that the binding event ocurrs in the central cavity. Based on the amount of guest it takes to saturate the host, one can use statistics to determine the binding affinity the host has for the guest. If the binding is strong you would need only a small excess of the guest. ITC measures the amount of heat absorbed or released as a solution of the host is titrated with a solution of the guest. From these measurements you can determine when the host has been saturated and determine the Enthalpy (ΔH), Entropy (ΔS), and Free Energy (ΔG). These thermodynamic constants explain what drives the binding events. And you can also calculate the binding constant (an equilibrium constant) from the free energy. Above are the results from 1H NMR and ITC titrations of the host with Na2SO4 to illustrate the explanations. We see that protons NH, B, and C are the ones which are most involved in binding and that the event is entropy driven. We determined that the strongest binding was observed for SO42- with an association constant of 2892 M-1 in water. While that number isn’t astronomically high–proteins can have much stronger affinities for biological molecules–it is relatively high for the fact that water is extremely competitive and these anions are strongly solvated. In comparison, look at a complex drug molecule and look up its solubility in water compared with sodium sulfate’s 28.1 g / 100 mL in water1 at room temperature. Acetaminophen (Tylenol) for example is only 1.4 g / 100 mL at room temperature,2 over 100 times less!
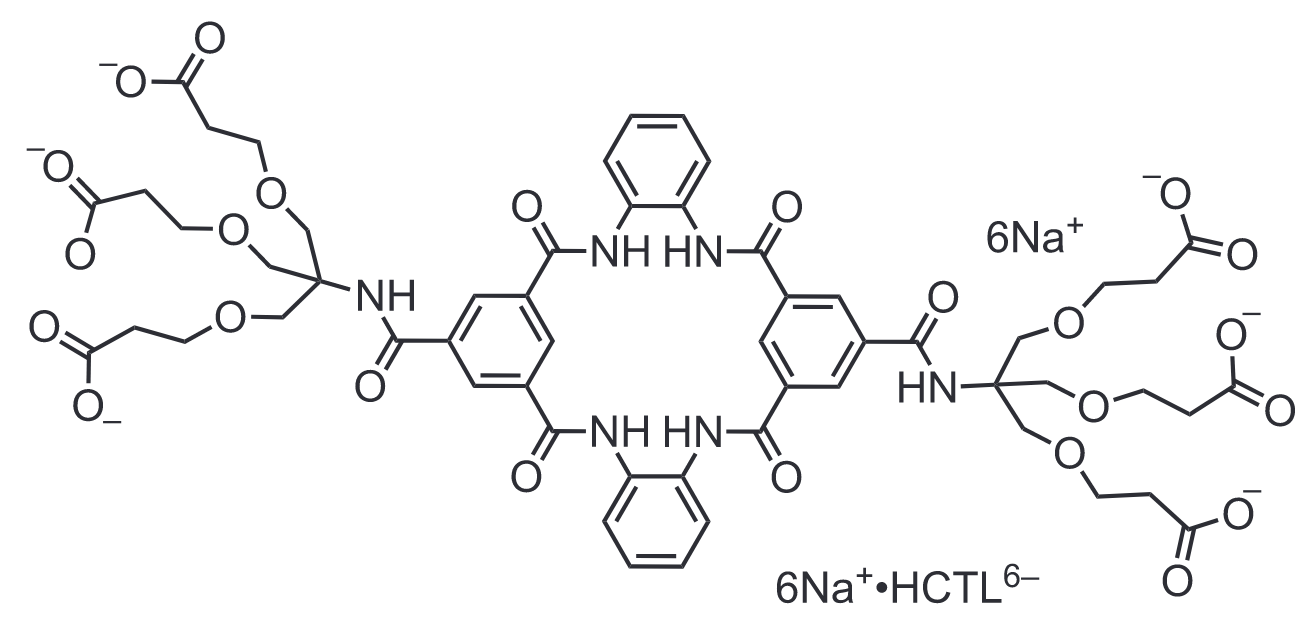 Chemdraw Structure of HCSL6-•6Na+
Chemdraw Structure of HCSL6-•6Na+
In the final work we did for the paper, we did the same experiments with a similar molecule that we synthesized. That molecule lacked the positively charged nitrogens and instead had a large water solubilization group attached where the nitrogen was alkylated (shown above). This molecule showed no appreciable binding at all to the same guests. This control molecule proves that to get this selectivity you must have suped up hydrogen bonding with the polarization of N‒H and C‒H bonds by nearby charges and electrostatic attraction caused by those charges. This gives us our “Charge-Assisted Hydrogen Bonding” tagline in the title.
Finally, thank you for reading through this whole summary. I look forward to posting more about my research in the future. If you aren’t someone with access to a university library or a company subscription to the relevent journal you can download the paper via the link below.
Paper Citation: Chem.-A Eur. J., 2025, e202501400 [Link]